A new publication of Stefanie is here!
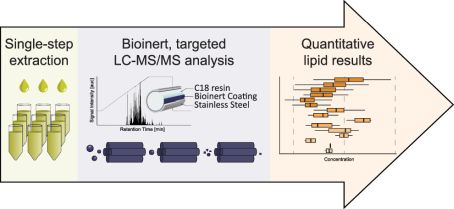
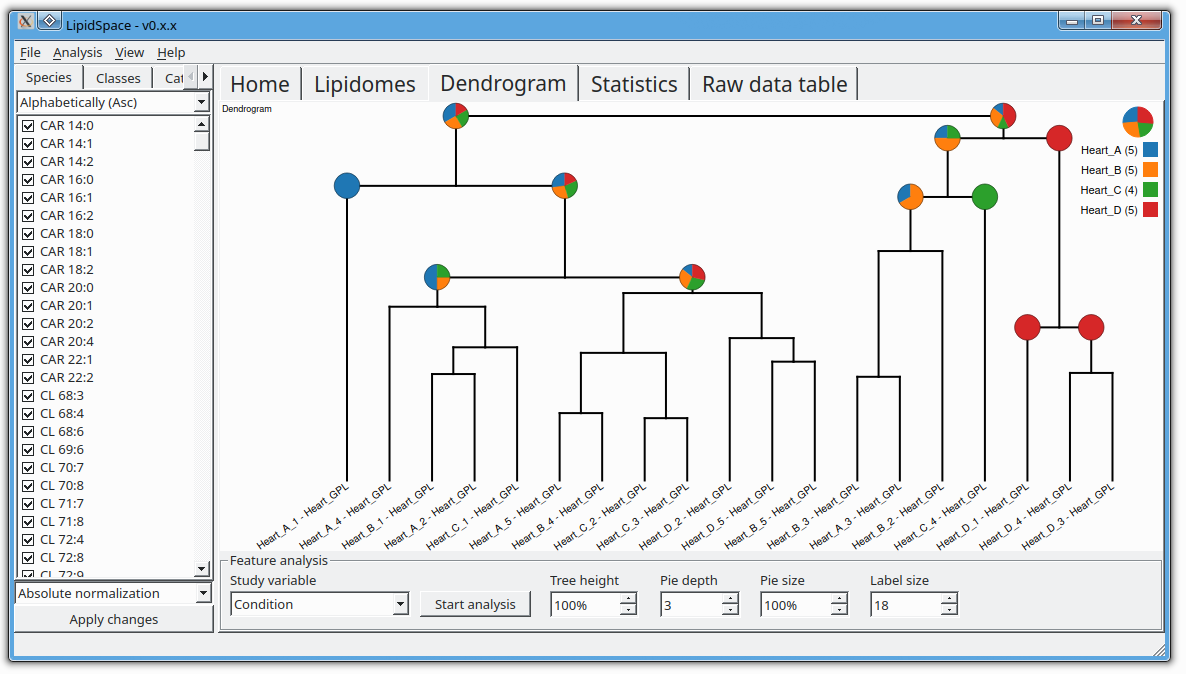
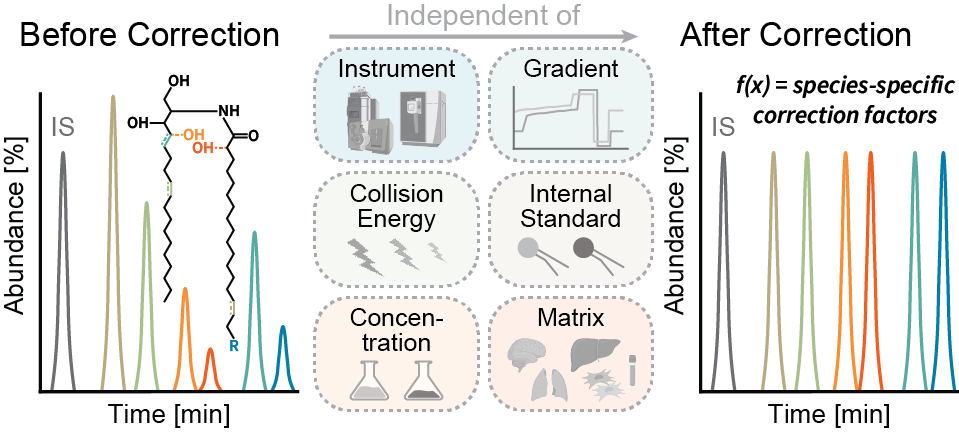
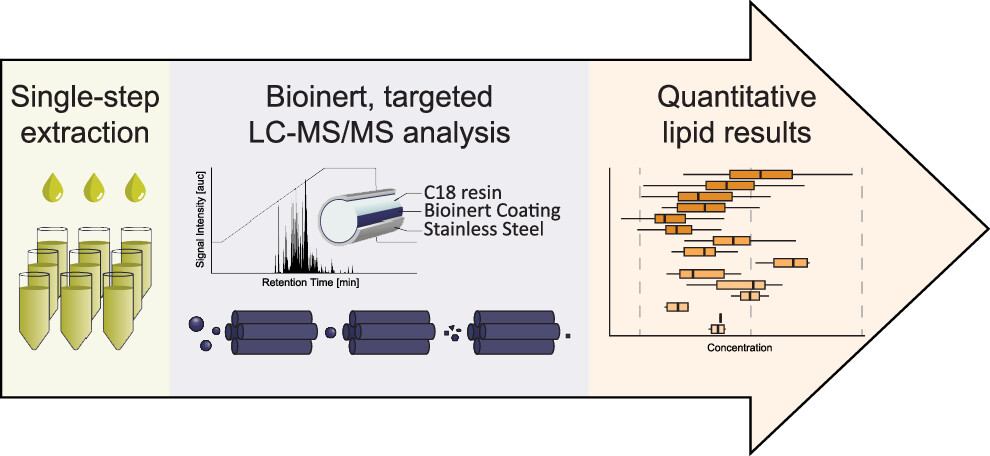
Signaling lipids play crucial roles in how cells function. However, it’s been difficult to monitor all these lipids at once because of their complexity and their chemical nature. We’ve developed a new, sensitive method using liquid chromatography-mass spectrometry (LC-MS/MS) that can accurately track a wide range of these lipids. Our method involves a straightforward sample extraction and uses specialized equipment to analyze 17 different classes of signaling lipids. This process is highly sensitive and consistent, allowing us to monitor 388 lipids in just 20 minutes. We tested our method on human plasma and successfully measured 307 different lipid molecules. We also studied changes in lipid signaling during platelet activation, finding many important regulatory pathways. This new technique opens up the possibility for detailed and comprehensive analysis of signaling lipids in complex biological systems.
Go to this publication!
Rubenzucker S, Manke MC, Lehmann R, Assinger A, Borst O, Ahrends R. A Targeted, Bioinert LC-MS/MS Method for Sensitive, Comprehensive Analysis of Signaling Lipids. Anal Chem. 2024 May 25. doi: 10.1021/acs.analchem.4c01388. Epub ahead of print. PMID: 38795073.