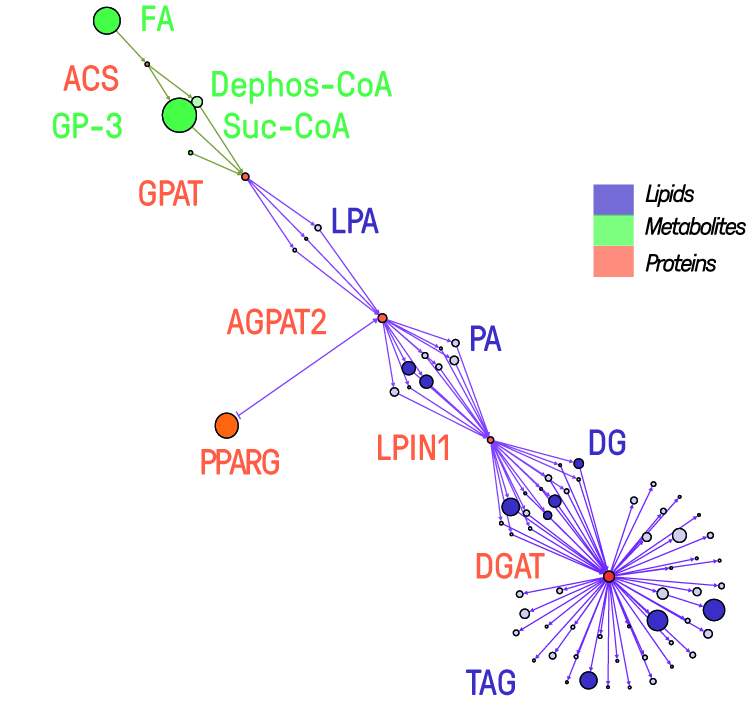
Lipidomics encompasses analytical approaches that aim to identify and quantify the complete set of lipids, defined as lipidome in a given cell, tissue, or organism, and their interactions with other molecules. Most lipidomics workflows are based on mass spectrometry and have been proven a powerful tool in system biology in concert with other Omics disciplines. Unfortunately, computational approaches for this relatively young discipline are limited and only accessible to some specialists. Search engines, quantification algorithms, visualization tools, and databases developed by the ‘Lipidomics Informatics for Life-Science’ (LIFS) initiative will provide a structured and standardized format for broad access to these specialized bioinformatics pipelines. Many medical challenges related to lipid metabolic alterations will be highly supported by such capacity-building. Within LIFS, we already provide access to several tools, workflows, tutorials, and training via a unified web portal (https://lifs-tools.org/).
Lipidomics encompasses analytical approaches that aim to identify and quantify the complete set of lipids, defined as lipidome in a given cell, tissue, or organism, and their interactions with other molecules. Most lipidomics workflows are based on mass spectrometry and have been proven a powerful tool in system biology in concert with other Omics disciplines. Unfortunately, computational approaches for this relatively young discipline are limited and only accessible to some specialists. Search engines, quantification algorithms, visualization tools, and databases developed by the ‘Lipidomics Informatics for Life-Science’ (LIFS) initiative will provide a structured and standardized format for broad access to these specialized bioinformatics pipelines. Many medical challenges related to lipid metabolic alterations will be highly supported by such capacity-building. Within LIFS, we already provide access to several tools, workflows, tutorials, and training via a unified web portal (https://lifs-tools.org/). This website uses cookies so that we can provide you with the best user experience possible. Cookie information is stored in your browser and performs functions such as recognising you when you return to our website and helping our team to understand which sections of the website you find most interesting and useful.