
The 8th Lipidomics Forum and 2nd International Lipidomics Society (ILS) Conference convened from August 27th to 30th, 2023. It provided a dynamic platform for researchers, scientists, and industry experts to delve into the latest advancements in lipidomics.
The Local Organizing Committee, headed by Robert Ahrends and his working group at the University of Vienna, successfully planned and organized the event. The conference schedule was thoughtfully created to cover the diverse interests and expertise of the attendees. The four-day event commenced with a series of workshops and an opening by Vice-Rector Manuela Baccarini.
Keynote speakers from academia and industry delivered exciting talks on emerging trends and challenges in lipidomics, laying the foundation for the ensuing discussions. The LIFS and LipiTUM workshops unfolded over the subsequent days, covering a spectrum of topics ranging from lipid profiling technologies and methodologies to the functional roles of lipids in health and disease. Participants discussed mass spectrometry techniques, chromatographic approaches, and bioinformatics tools employed in lipidomics research.
The Forum and the Conference also provided a unique platform for early-career scientists and graduate students to present their research through dedicated sessions and poster presentations. This initiative put together the next generation of lipidomics researchers and promoted a collaborative spirit within the scientific community.
In addition to the scientific program, the conference facilitated networking opportunities, allowing participants to establish connections with peers, mentors, and industry representatives.
The conference concluded on August 30th, 2023, and left a lasting impact on the lipidomics community. The organizers thank all attendees and are grateful for the industry sponsorship, making this meeting a vibrant event. We are looking forward to welcoming you again from the 14th to the 17th of September in 2025.
Pics by Martin Schaier.




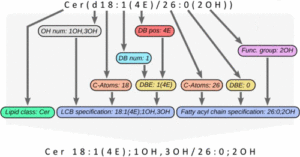 The next version of GOSLIN is there! Goslin is the first grammar-based computational library for the recognition/parsing and normalization of lipid names following the hierarchical lipid shorthand nomenclature. The new version Goslin 2.0 implements the latest nomenclature and adds an additional grammar to recognize systematic IUPAC-IUB fatty acyl names as stored, e.g., in the LIPID MAPS database and is perfectly suited to update lipid names in LIPID MAPS or HMDB databases to the latest nomenclature. Goslin 2.0 is available as a standalone web application with a REST API as well as C++, C#, Java, Python 3, and R libraries. Importantly, it can be easily included in lipidomics tools and scripts providing direct access to translation functions. All implementations are open source.
The next version of GOSLIN is there! Goslin is the first grammar-based computational library for the recognition/parsing and normalization of lipid names following the hierarchical lipid shorthand nomenclature. The new version Goslin 2.0 implements the latest nomenclature and adds an additional grammar to recognize systematic IUPAC-IUB fatty acyl names as stored, e.g., in the LIPID MAPS database and is perfectly suited to update lipid names in LIPID MAPS or HMDB databases to the latest nomenclature. Goslin 2.0 is available as a standalone web application with a REST API as well as C++, C#, Java, Python 3, and R libraries. Importantly, it can be easily included in lipidomics tools and scripts providing direct access to translation functions. All implementations are open source. 





